April 2021
Robotics Rehabilitation Device for ICU Patients - Winner of euRobotics Technology Transfer Award
VEMO (Very Early Mobilization), the first safe, AI-powered human-robot therapy in intensive care, was the winner of the euRobotics Technology Transfer Award on April 15, 2021, at the European Robotics Forum (ERF).
VEMO is the product of cooperation among Reactive Robotics GmbH (RR), the TUM Munich School of Robotics and Machine Intelligence (MSRM), and the Schön Klinik Bad Aibling (SKBA) Neurological (Early) Rehabilitation Specialist Center. The motivation behind this effort was to develop a sustainable, intuitively applicable long-term solution to the challenge of providing ICU patients sufficient rehabilitation care for optimal recovery.
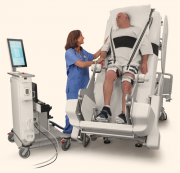
VEMO does not require any manual patient movement or transfer. It allows the patient to maximize their individual movement by automatically detecting the patient’s (dis-)ability level and providing the minimum amount of support necessary for the patient to achieve the therapy goals. Furthermore, even lower-qualified staff such as nurse-assistants are able to use VEMO to perform therapy, so that nurses, who are in dire shortage, can perform other important duties. Therapists can simply decide on the therapy paradigm and leave the continuous adjustment of treatment settings throughout the therapy sessions to the machine which can adjust within seconds to each individual patient’s abilities and disabilities.
and providing the minimum amount of support necessary for the patient to achieve the therapy goals. Furthermore, even lower-qualified staff such as nurse-assistants are able to use VEMO to perform therapy, so that nurses, who are in dire shortage, can perform other important duties. Therapists can simply decide on the therapy paradigm and leave the continuous adjustment of treatment settings throughout the therapy sessions to the machine which can adjust within seconds to each individual patient’s abilities and disabilities.
A subgroup of patients that will particularly benefit from this rehabilitation device are neurological patients suffering from injuries such as stroke, spinal cord injury, or traumatic brain injury. Besides neurological patients, first tests with Covid-survivors were also very promising.
VEMO is CE certified as of fall 2019 by TÜV Süd and was commercially launched in the summer of 2020. Several hospitals in Germany, Austria, Switzerland, and Italy are already using the system. FDA 510(k) exempt status has been confirmed by the Federal Drug Administration in the US. Market entry in the USA is planned for the end of 2021. Reactive Robotics GmbH plans to place 20 VEMO systems on the European market in 2021, 60 systems on the US market and EU in 2022, and 150 systems in 2023. Assuming each VEMO system can treat up to 6 patients per day and an ICU patient’s average length of stay is 7 days, over 18,000 patients could benefit from this robotics assistive rehabilitation device in 2022.
About euRobotics Technology Transfer award:
The euRobotics Technology Transfer Award, now in its 17th edition, is coordinated by Dr. Werner Kraus, Head of Department Robot and Assistive Systems at Fraunhofer IPA. The award honors outstanding examples of technology transfer in robot technology and automation that result from cooperative efforts between research and industry. Successful technology transfer describes the process of converting scientific findings from research laboratories into innovative products, processes, and services by the commercial sector.
The jury committee consists of: Herman Bruyninckx (KU Leuven), Rainer Bischoff (X, The Moonshot Factory), Fariba Khatami (EUnited aisbl), Nicola Tomatis (BlueBotics SA), and Georg von Wichert (Siemens AG).
Industrious Brussels EU District, Avenue des Arts 6-9, 1210 Brussels, Belgium, +32 490 57 57 65
Transparency Register number: 0289344948-82














































































































